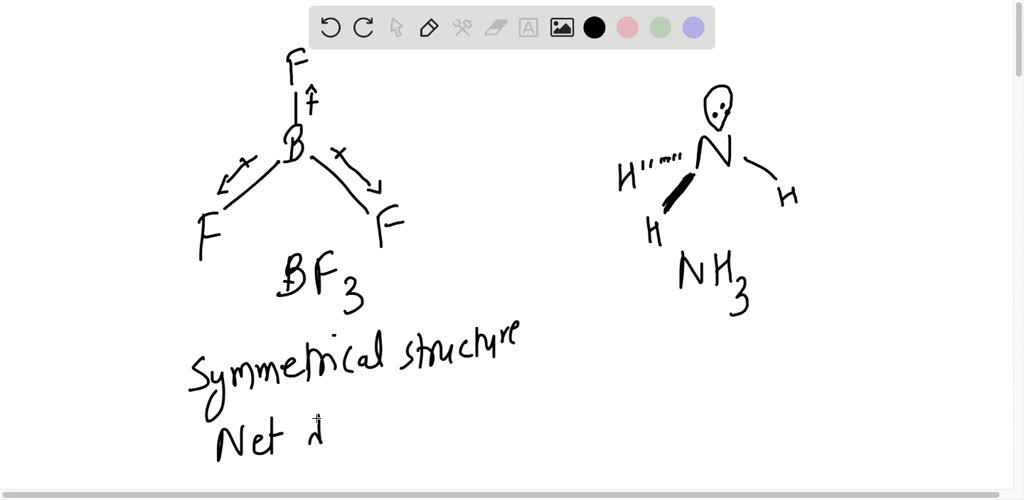
Which out of the following pairs has dipole moment and why ? nbsp; i] BF3 AND NF3 nbsp; ii] CO2 AND H2O

Which have a molecular dipole moment? (Select all that apply.) 1. BF3 2. SF4 3. BrF3 4. NF3 5. CF4 | Homework.Study.com
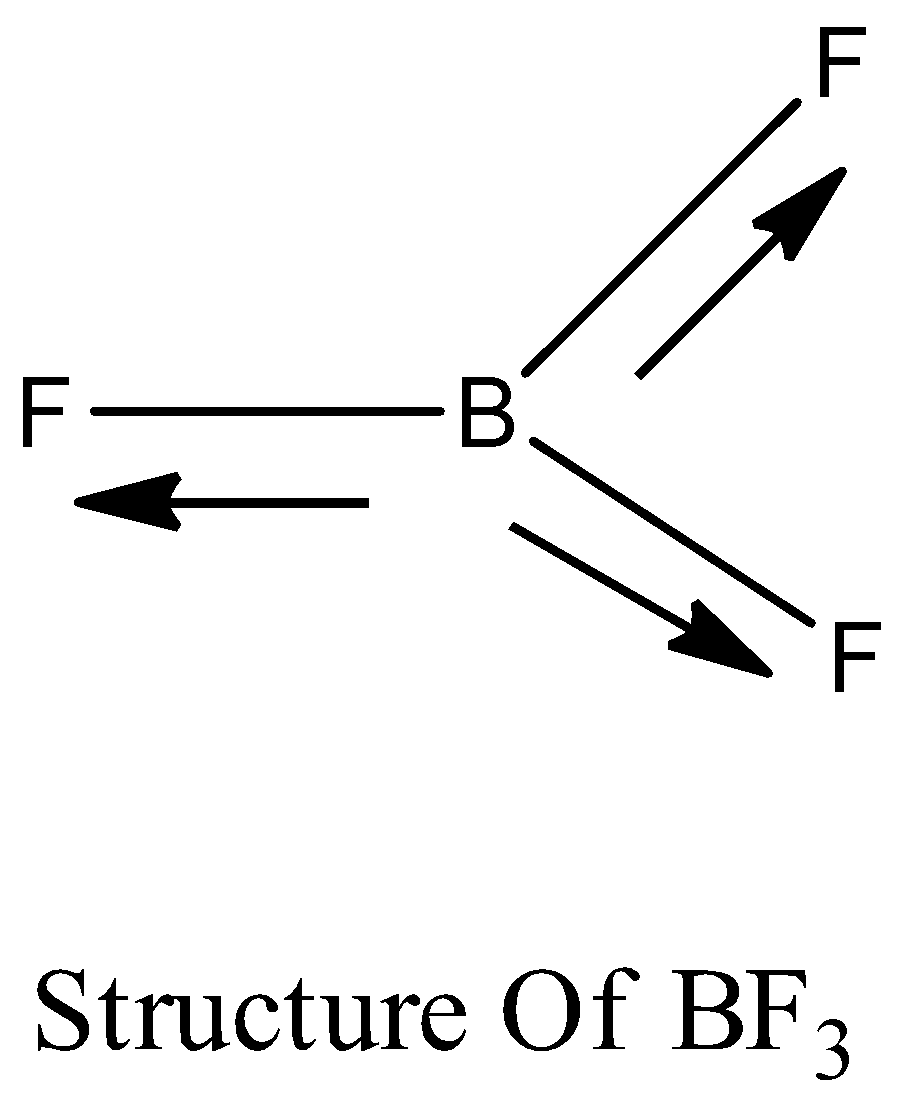
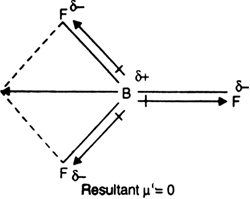
Boron trifluoride $(B{F_3})$ has no dipole moment $(\\mu = 0D)$. Explain how this observation confirms the geometry of $B{F_3}$ predicted by VSEPR theory.
Dipole moment in case of BF3 is zero. Explain. - Sarthaks eConnect | Largest Online Education Community
Which out of the following pairs has dipole moment and why ? nbsp; i] BF3 AND NF3 nbsp; ii] CO2 AND H2O

![Odia] Among C Cl4, BF3, NH3 and CO2, Which one has net dipole moment Odia] Among C Cl4, BF3, NH3 and CO2, Which one has net dipole moment](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/11812254.webp)