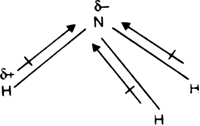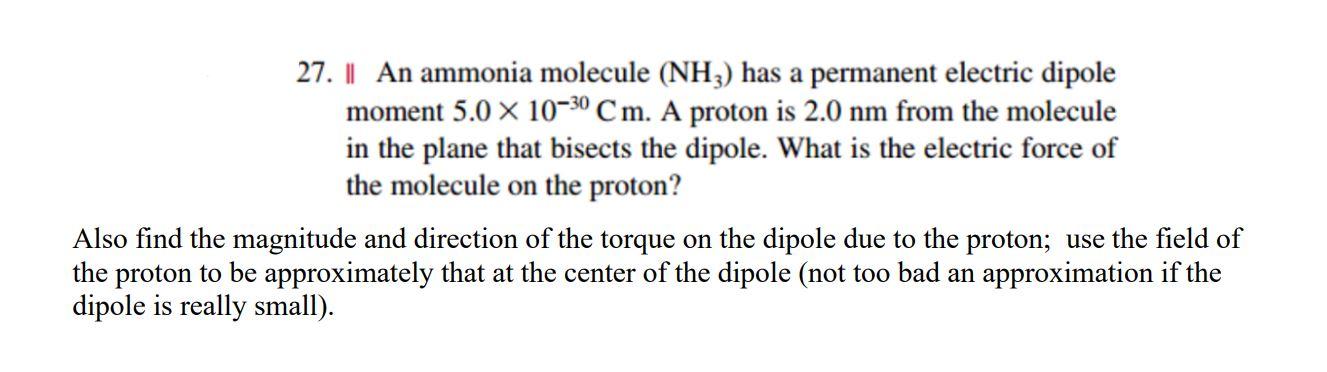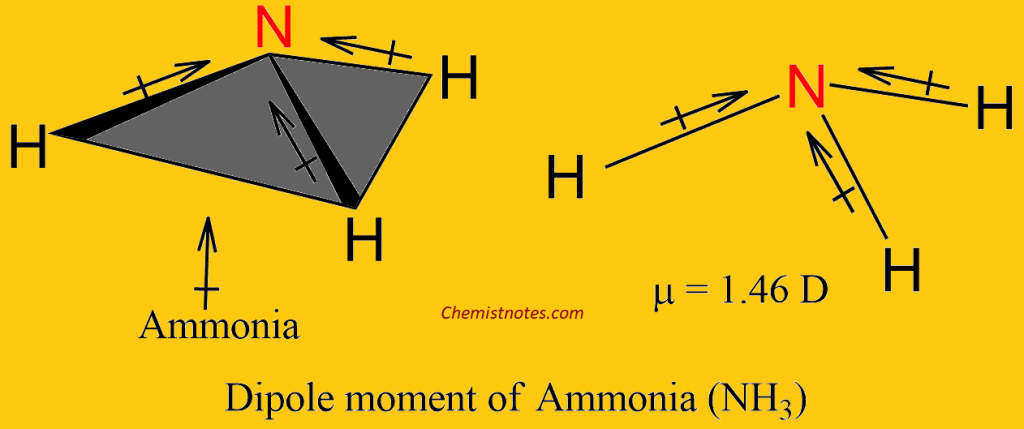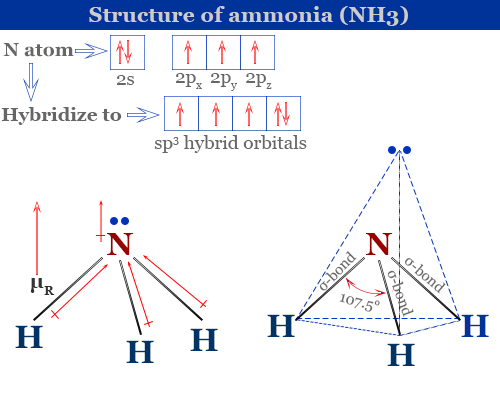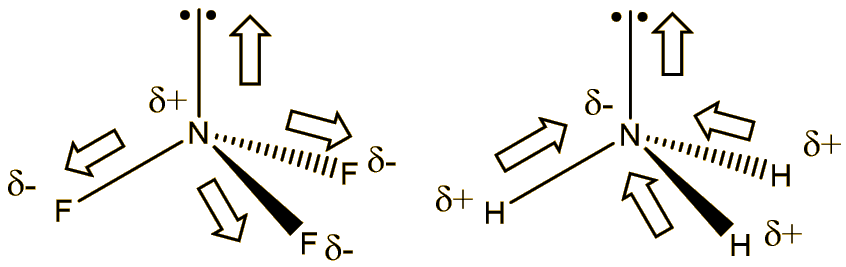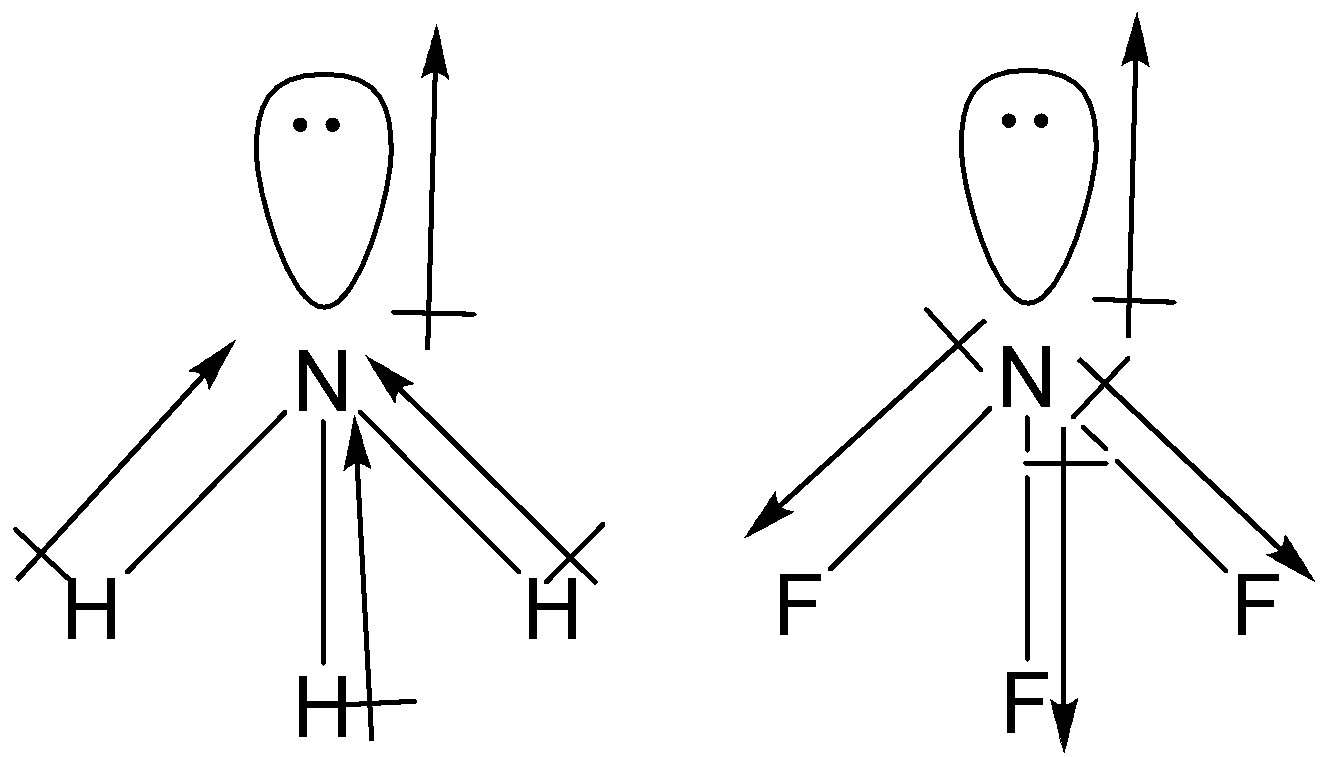
What is the correct dipole moment of $N{{H}_{3}}$ and $N{{F}_{3}}$ respectively?(A)- $4.90\\times {{10}^{-30}}$ C m and $0.80\\times {{10}^{-30}}$ C m(B)- $0.80\\times {{10}^{-30}}$ C m and $4.90\\times {{10}^{-30}}$ C m(C)- $4.90\\times {{10}^{-30}}$ C

Can you explain why NH3 has such a large dipole moment compared with NF3? Show work. | Homework.Study.com
17. The electronegativity difference between N F is greater than that between N H. Yet the dipole moment of NH3 is 1.5D. This is because: 1. In NH3 the atomic dipole and

Effect of on dipole moments of CO, O3, NH3 molecules Dipole moment |P e... | Download Scientific Diagram
Which out of ammonia (NH3) and NF3 has higher dipole moment and why? - Sarthaks eConnect | Largest Online Education Community