The dipole moment of chlorobenzene is `1.6D`m The expected dipole moment of meta-dichlorobenzene is: - YouTube
The Dipole Moments of Chlorobenzene, Monochlorocyclopropane, and 1,2-Dichlorocyclopropane, with a Calculation of the Exterior Valence Angle of the Cyclopropane Ring1 | Journal of the American Chemical Society

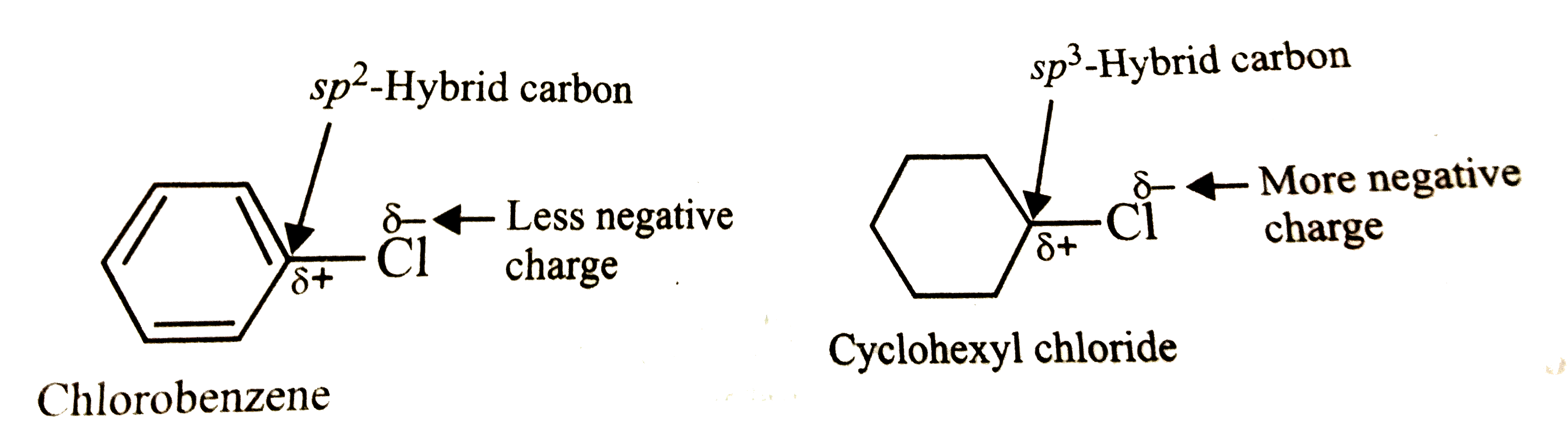
Explain why the dipole moment of chlorobenzene is lower than cyclohexyl chloride ? – The Unconditional Guru
e dipole moment of chlorobenzene is 1.5 D. Theighest dipole moment will be shown by(1) 1, 2 dichlorobenzene (2) 1, 2, 3 trichlorobenzene(3) 1, 2, 3, 4 tetrachlorobenzene(4) 1, 2, 3, 4, 5 pentacobenzene

Solve this: (d) Reactivity-Be Li K Cs The dipole moment of chlorobenzene 1 5 - Chemistry - The Solid State - 12295951 | Meritnation.com

Determine if the given species has a permanent dipole moment. Chlorobenzene, C6H5Cl | Homework.Study.com

The dipole moment of chlorobenzene is 1.5D . Calculate dipole moment of 1,2,3,5 - tetrachlorobenzene.

Give reasons:(i) C - Cl bond length in chlorobenzene is shorter than C - Cl bond length in CH3 - Cl .(ii) The dipole moment of chlorobenzene is lower than that of

Explain why the dipole moment of chlorobenzene is lower than cyclohexyl chloride ? – The Unconditional Guru

Pls Explain This Sum With Formula And How To Find Dipole Moment In Benzene Like Compounds Explain Me With Example - Chemistry - Stoichiometry - 10436977 | Meritnation.com
![The dipole moment of chlorobenzene is 1.73 D. The dipole moment of p di chlorobenzene is expected to be [CPMT 1991] (a) 3.46 D (c) 1.73 D (b) 0.00 ID (d) 1.00 D The dipole moment of chlorobenzene is 1.73 D. The dipole moment of p di chlorobenzene is expected to be [CPMT 1991] (a) 3.46 D (c) 1.73 D (b) 0.00 ID (d) 1.00 D](https://toppr-doubts-media.s3.amazonaws.com/images/1847473/f410b4fb-27a2-42b1-8a89-a5859a0c6cf0.jpg)
The dipole moment of chlorobenzene is 1.73 D. The dipole moment of p di chlorobenzene is expected to be [CPMT 1991] (a) 3.46 D (c) 1.73 D (b) 0.00 ID (d) 1.00 D

Explain why (i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (i... - YouTube
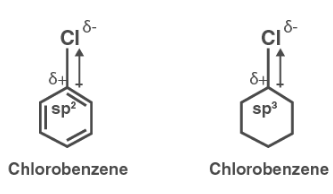
Explain why (i) the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride? (ii) alkyl halides, though polar, are immiscible with water? (iii) Grignard reagents should be prepared under anhydrous