
Although flourine is more electronegative than nitrogen, the resultant dipole moment of ammonia is greater than nitrogen trifluoride Explain - Chemistry - Chemical Bonding and Molecular Structure - 6229927 | Meritnation.com
Why is the moment dipole for ammonia (1.64D substantially higher than that of ammonia trifluoride (0.24D) although the latter contains the highly electronegative flourine? - Quora
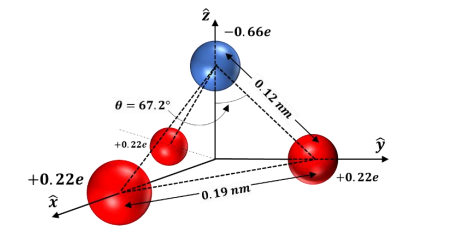
What is the correct dipole moment of $N{{H}_{3}}$ and $N{{F}_{3}}$ respectively?(A)- $4.90\\times {{10}^{-30}}$ C m and $0.80\\times {{10}^{-30}}$ C m(B)- $0.80\\times {{10}^{-30}}$ C m and $4.90\\times {{10}^{-30}}$ C m(C)- $4.90\\times {{10}^{-30}}$ C
Why does ammonia has induced dipole induced dipole attraction? Whats the difference between induced dipole induced dipole, dipole dipole and bonds? - Quora
Why is the moment dipole for ammonia (1.64D substantially higher than that of ammonia trifluoride (0.24D) although the latter contains the highly electronegative flourine? - Quora

Is NH3 polar Or Nonpolar? - nh3 Intermolecular Forces, nh3 charge, nh3 bond angle, detailed facts? - chemwhite.com
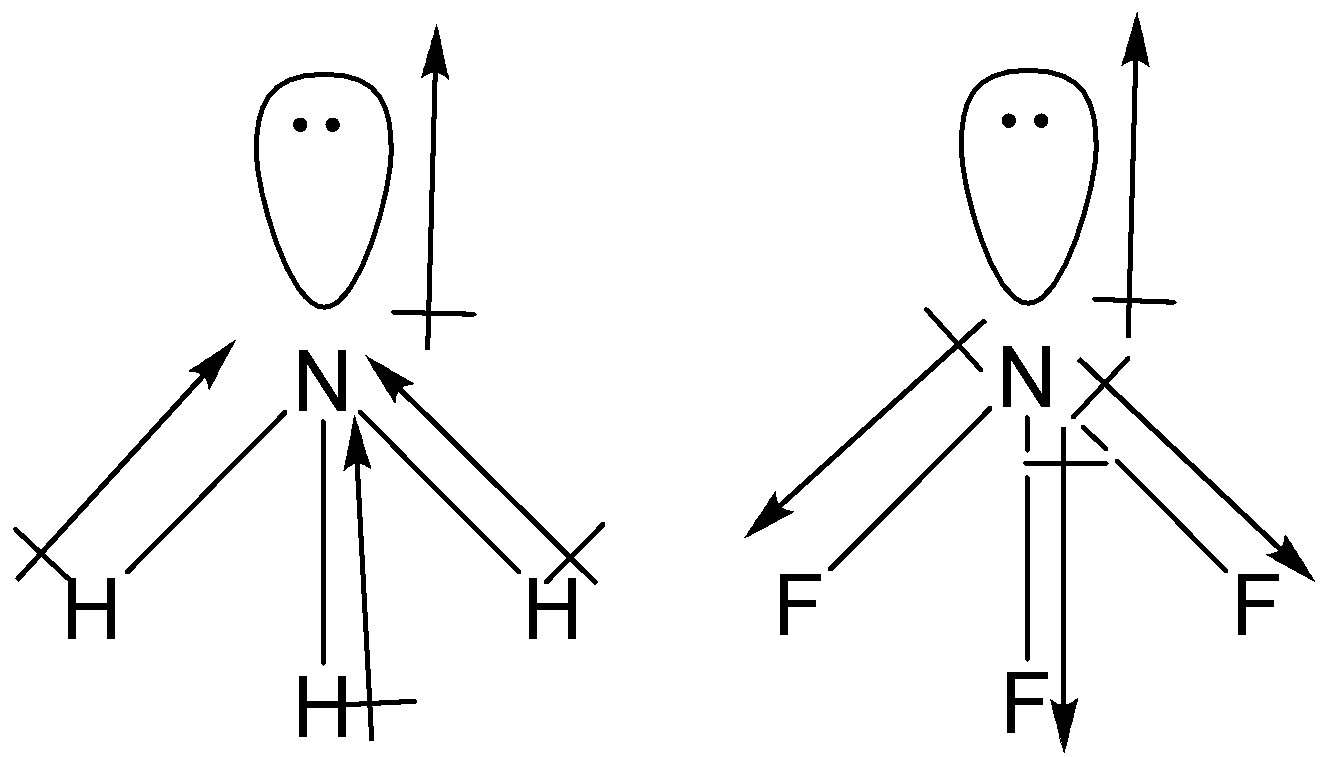
Which out of ammonia (NH3) and NF3 has higher dipole moment and why? - Sarthaks eConnect | Largest Online Education Community
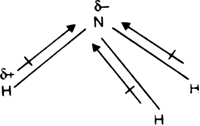
17. The electronegativity difference between N F is greater than that between N H. Yet the dipole moment of NH3 is 1.5D. This is because: 1. In NH3 the atomic dipole and









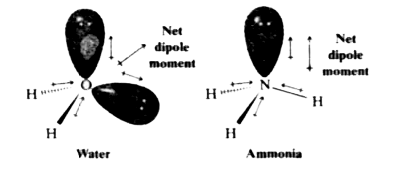


![Which out of \\[N{H_3}\\] and $N{F_3}$ has a higher dipole moment and why? Which out of \\[N{H_3}\\] and $N{F_3}$ has a higher dipole moment and why?](https://www.vedantu.com/question-sets/a32afb56-fc67-43ca-8a5c-e06f6fe2b15f5994708008049533229.png)